Under pressure from glaucoma
Here, I present two challenging glaucoma cases and their management.
Case A
A healthy 34-year-old male was referred to me for a second opinion. He had a complex history of presumed idiopathic early onset corneal endothelial dystrophy, failed corneal transplant surgeries, secondary glaucoma with failed trabeculectomy and micropulse and cataract surgery with iris segment implants for Urrets-Zavalia syndrome. He had no other medical history of note. Vision at review was hand motion in the affected eye (left eye) and 6/15 in the other eye.
Gonioscopy with topical glycerol showed evidence of anterior synechiae in all quadrants. Visibility through the cornea was limited, with iris segments and his intraocular lens just visible (Fig 1). There was no view of the fundus. Visual field testing was impossible due to his poor vision. Optical coherence tomography (OCT) showed gross thinning of the optic nerve (Fig 2). Intraocular pressure (IOP) was elevated at 38mmHg on maximal medical treatment, which included Dortimopt (dorzolamide and timolol maleate) twice a day (BD), brimonidine BD, latanoprost daily and oral Diamox (acetazolamide) 125mg BD.
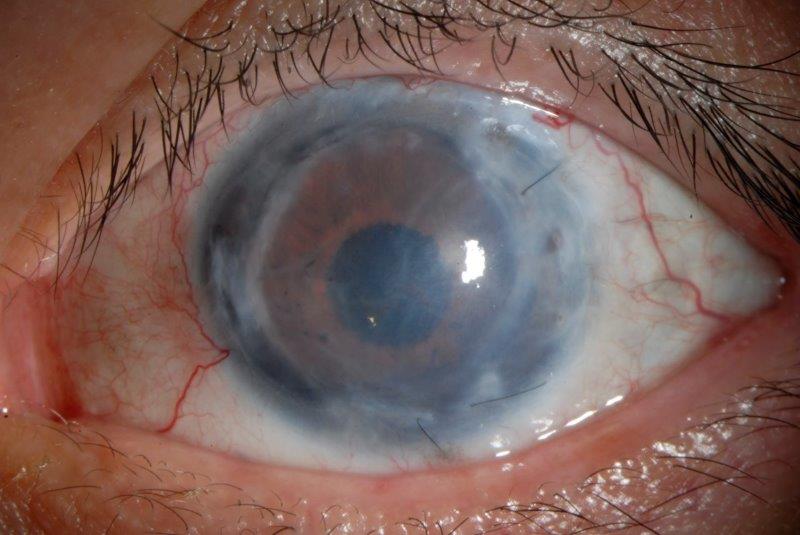
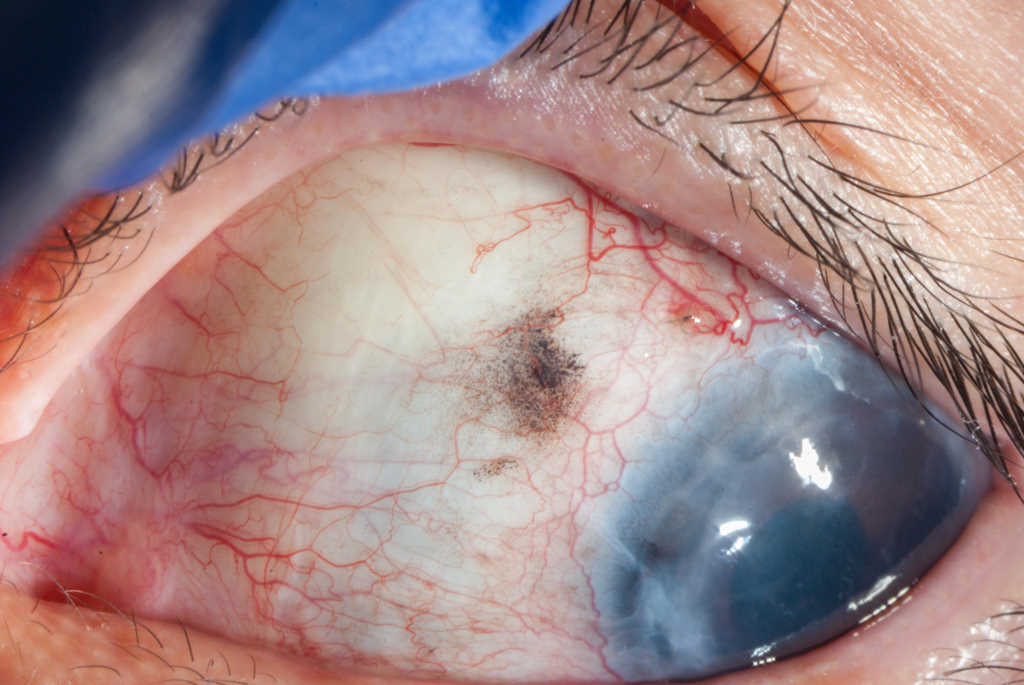
Fig 1. A) Anterior segment prior to surgery showed an opaque cornea secondary
to corneal graft failure with folds in Descemet’s membrane
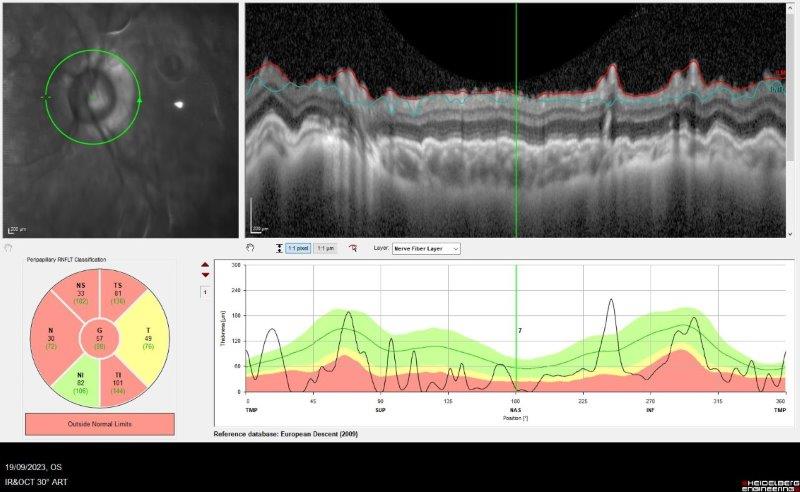
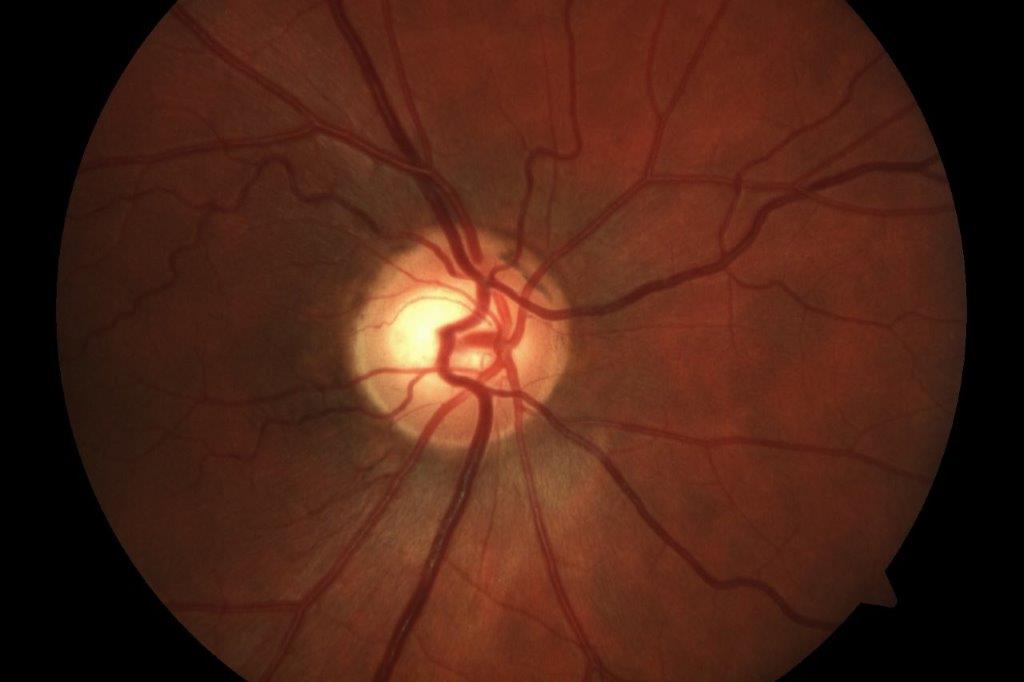
Fig 2. OCT showing gross attenuation of retinal nerve fibre layer
The patient underwent surgery with a sulcus-placed infero-temporal Paul glaucoma drainage tube. Surgery was challenging due to poor corneal visibility. Postoperatively, his IOP was 8mmHg, but subsequently elevated to 48mmHg three weeks later, requiring restarting glaucoma medications. Subsequently, early intraluminal stent removal was performed, resulting in hypotony of 2mmHg and anterior chamber shallowing. This was managed with atropine and cessation of topical steroids. At three months post-operative follow-up his IOP was 8mmHg off all glaucoma medications, and he underwent further corneal transplantation surgery. His IOP remains well controlled at 8mmHg, seven months post-surgery (Fig 3).
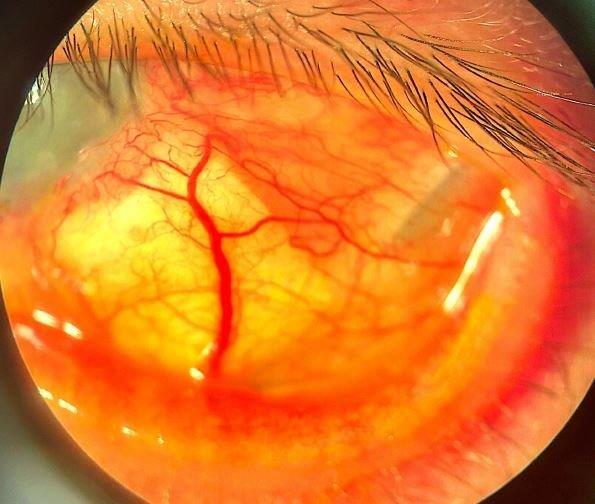
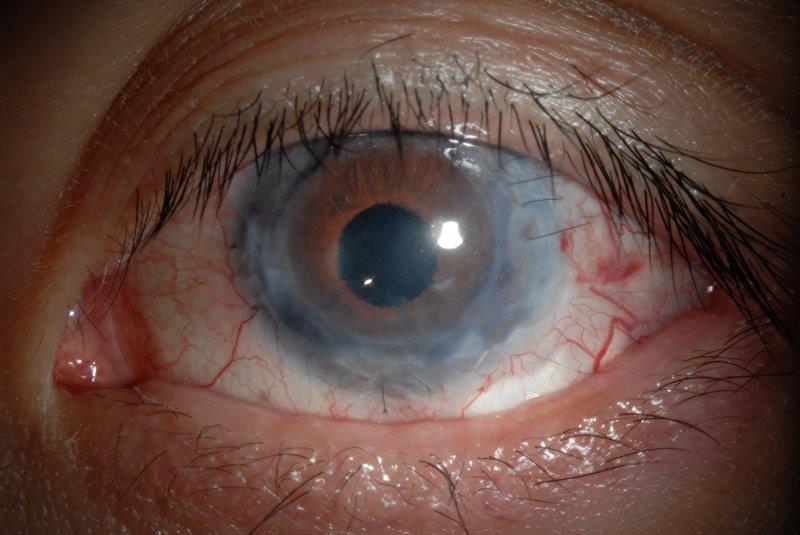
Fig 3. A) Anterior segment photo following surgery shows an inferior temporal placed sulcus drainage tube implant, with evidence of a scleral patch graft, and a distal drainage bleb. B) Good cosmesis on primary gaze and the corneal transparency is improved (right)
Discussion
The relationship between glaucoma and the anterior segment is very interdependent. Glaucoma treatments have been associated with corneal graft failure1 and there is an increased risk of corneal transplant failure in patients with glaucoma2. In this case, it was imperative to manage his IOP prior to a repeat corneal graft surgery to minimise the risk of corneal graft failure. The five-year graft failure risk is 7% in patients with corneal endothelial dystrophy. Prior glaucoma surgery and preoperative IOP-lowering medications were associated with a 58% incidence of five-year graft failure. The 10-year graft failure risk is more than doubled in patients on glaucoma treatment compared to patients without glaucoma3.
So, what is it about glaucoma that increases the risk of corneal transplant failure? Glaucoma topical medications increase inflammatory cells in the conjunctiva and limbus, which may lead to graft rejection. Beta blockers and carbonic anhydrase inhibitors disrupt endothelial cell function and cholinergic agents can disrupt the blood-aqueous barrier and increase inflammation1. Furthermore, anti-metabolites such as fluorouracil, used during glaucoma surgery, may be toxic to the corneal endothelium4. Filtration and tube surgery can cause a transient breakdown of the blood-aqueous barrier and endothelial damage from direct contact with the cells, further contributing to graft failure.
This case is challenging for many reasons. There was no space in the superior fornix for tube placement, as the previous failed trabeculectomy had resulted in significant scleral thinning and conjunctival scarring. The decompensated corneal graft limited the visualisation of the tube during the time of the surgery, which increased the risk of iris root damage and incorrect tube positioning. An inferotemporal-placed tube may be associated with poor cosmesis, diplopia, tube erosion and bleb dysesthesia. A sulcus placement for the tube was preferred to reduce the risk of corneal endothelial trauma and future risk of graft failure. Finally, measuring IOP is challenging (due to corneal astigmatism) in graft patients, coupled by poor visualisation of the optic disc and unreliable visual field results, which are integral to monitoring glaucoma progression.
This patient had a significant hypertensive postoperative phase. It is typically not recommended to remove the intraluminal stent in glaucoma drainage tube surgery prior to three months, due to the risk of hypotony. However, it was considered necessary in this patient to avoid the risk of further optic disc damage. Fortunately, careful augmentation of his postoperative drop regimen was sufficient to avoid further complications.
It is important for eyecare professionals to monitor such patients closely to minimise potential progression of glaucoma and maintain the health of the corneal transplant. Understanding the association between glaucoma and anterior segment surgery should trigger earlier glaucoma interventions to promote the long-term success of corneal transplant surgery.
Case B
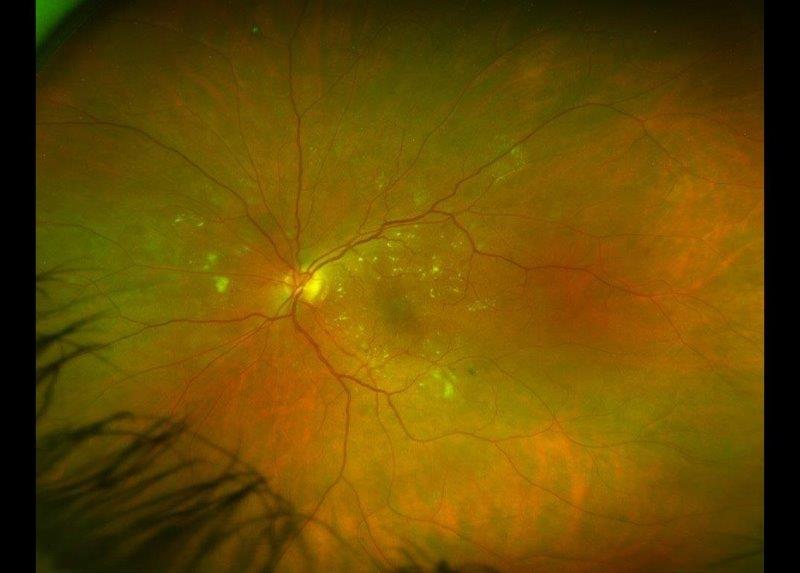
A 59-year-old Indian female, with a background of breast cancer in remission, was referred from the uveitis service for glaucoma management. She initially presented via the emergency eye clinic with cloudy vision, photophobia and pain in the left eye. The findings at presentation included counting-fingers vision, IOP of 40mmHg, corneal oedema, low-grade non-granulomatous uveitis (3+ cells) with no significant retinitis or choroiditis. A tentative diagnosis of hypertensive uveitis was made and she was managed with Combigan (brimonidine/timolol) twice a day, topical steroids and cycloplegics. Uveitis screening results were negative and she was given a therapeutic trial of valaciclovir and oral prednisone. Subsequently, her IOP increased to 56mmHg, which necessitated Diamox (acetazolamide) treatment. She was referred for a glaucoma opinion due to progressive visual field defects and thinning of the retinal nerve fibre layer (Fig 4 and Fig 5). Gonioscopy showed evidence of angle closure with peripheral anterior synechiae in all quadrants. There was a dense cataract. Visual field testing showed gross constriction (Fig 6).
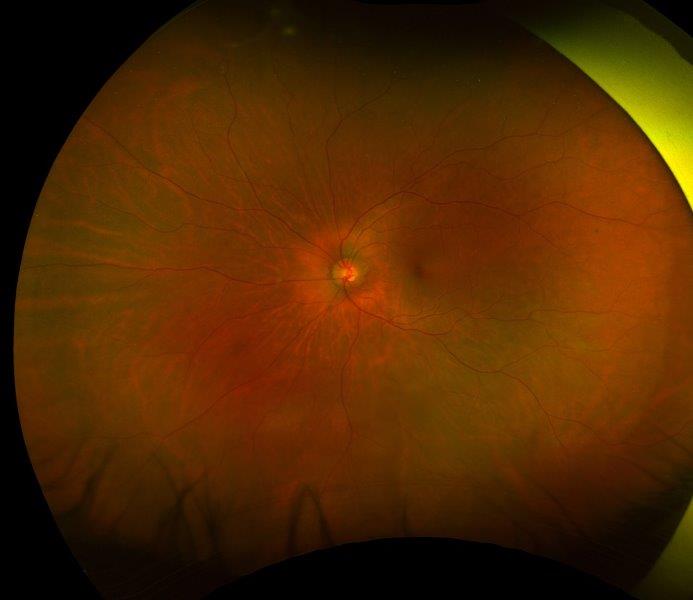
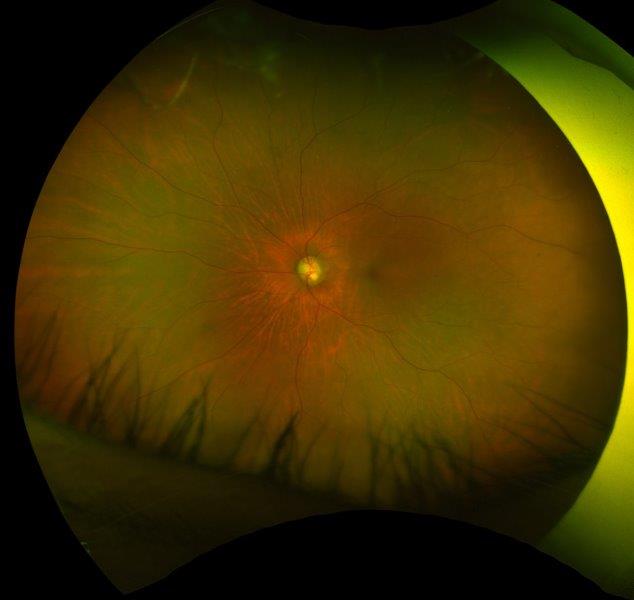
Fig 4. Posterior segment photo showing significant disc thinning and pallor. A) is at presentation, and B) is at two months later (right)
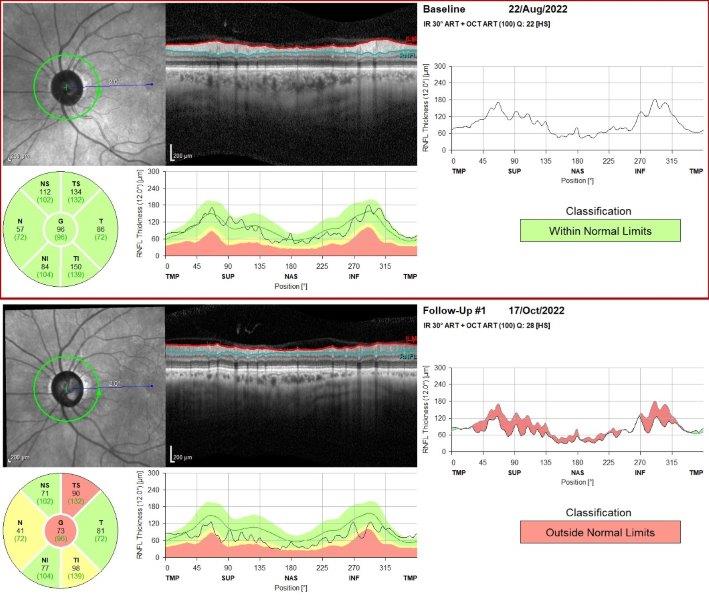
Fig 5. OCT showing gross attenuation of retinal nerve fibre layer within two months
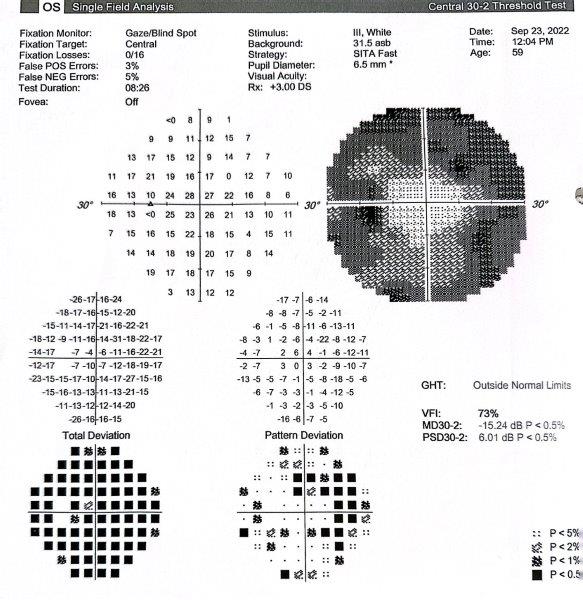
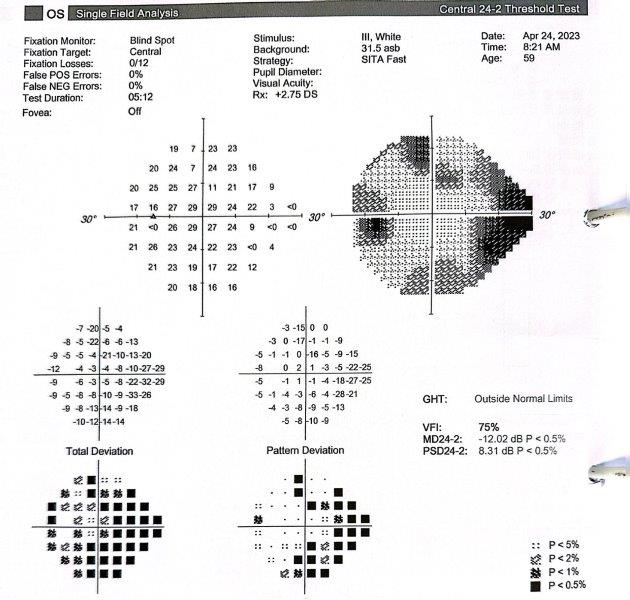
Fig 6. Visual field showing A) gross loss of peripheral vision, which B) partially recovered following surgery
This is a case of uveitis with secondary glaucoma. Due to her cataracts and advanced glaucomatous neuropathy, the patient underwent cataract surgery combined with a sulcus drainage tube implanted in the superior temporal quadrant. Six months later, her IOP was 10mmHg on no treatment and she demonstrated partial recovery of optic nerve function.
Discussion
The pathophysiology of uveitic glaucoma is guided by the status of the drainage angle. Angle closure from posterior synechiae can lead to pupil block and iris bombe, while anterior synechiae can lead to inflammatory iridotrabecular contact. Secondary pressure elevation on open angles may be due to 1) mechanical obstruction by cellular debris, 2) hyper-secretion from prostaglandin mediated vascular hyperpermeability, 3) trabecular inflammation and sclerosis and 4) trabecular obstruction by a hyaline membrane5. Steroid-induced glaucoma typically occurs over weeks to months and usually corrects with cessation of treatment.
The initial challenge is to determine the glaucoma’s cause. The differential diagnosis of uveitis glaucoma in the presence of pigment includes steroid-induced glaucoma, pigmentary glaucoma and, in some cases, advanced angle-closure glaucoma. Uveitic eyes have increased pigment in the drainage angle, usually from shedding of pigment from the iris and broken synechiae.
Surgical management was considered in this patient as there were concerns of rapid progressive optic neuropathy and failed medical treatment. Her listing IOP was 44mmHg on maximum medical treatment. Broadly speaking, the initial choices of surgery included 1) cataract surgery as a standalone procedure, 2) cataract surgery plus trabeculectomy, or 3) cataract surgery plus drainage tube positioned in the sulcus. The first principle in managing uveitic glaucoma includes controlling the inflammation, when possible, prior to surgery. This patient was started on systemic prednisone 40mg daily, one week prior to surgery, which was later tapered by 5mg per day per week postoperatively.
In my experience, glaucoma drainage devices work better in uveitic glaucoma and in patients who have pigmented skin who are more prone to scarring. Deep sclerectomy and trabeculectomy augmented with mitomycin-C can achieve pressure control in uveitic glaucoma patients; however, intensive monitoring is necessary due to increased risk of hypotony and conjunctival scarring.
In this patient, I opted for a combined cataract and glaucoma drainage tube operation, as she had dense anterior synechiae. It would have made anterior chamber placement challenging, due to a greater risk of corneal decompensation from an anterior-placed tube, and potential risk of iris root damage. Due to the presence of cataract, the logical choice was to perform cataract surgery with a sulcus tube placement. I generally avoid performing combined surgery, but in this case the rapid decline in visual function meant there was limited time for a staged procedure. Fortunately, there was adequate reserve in her visual function, which showed some degree of recovery of peripheral vision following surgery.
It is important for eyecare professionals to be aware of the multiple factors leading to uveitic glaucoma. These patients are at higher risk of surgical complications and require careful preoperative planning and more intensive follow-up to maximise success.
References
- Greenlee E, Kwon Y. Graft failure: III. Glaucoma escalation after penetrating keratoplasty. Int Ophthalmol. 2008 Jun;28(3):191-207.
- Sugar A, Tanner J, Dontchev M, Tennant B, Schultze R, Dunn S, Lindquist T, Gal R, Beck R, Kollman C, Mannis M, Holland E; Cornea Donor Study Investigator Group. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009 Jun;116(6):1023-8.
- Dunn S, Gal R, Kollman C, Raghinaru D, Dontchev M, Blanton C, Holland E, Lass J, Kenyon K, Mannis M, Mian S, Rapuano C, Stark W, Beck R; Writing committee for the Cornea Donor Study Research Group. Corneal graft rejection 10 years after penetrating keratoplasty in the cornea donor study. Cornea. 2014 Oct;33(10):1003-9.
- Skuta G, Beeson C, Higginbotham E, Lichter P, Musch D, Bergstrom T, Klein T, Falck F Jr. Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology. 1992 Mar;99(3):438-44.
- Siddique S, Suelves A, Baheti U, Foster C. Glaucoma and uveitis. Surv Ophthalmol. 2013 Jan-Feb;58(1):1-10.

Dr Divya Perumal is a New Zealand- and UK-trained ophthalmologist and University of Auckland optometry graduate. Specialising in glaucoma, cataract and pterygium, she consults for Te Whatu Ora.