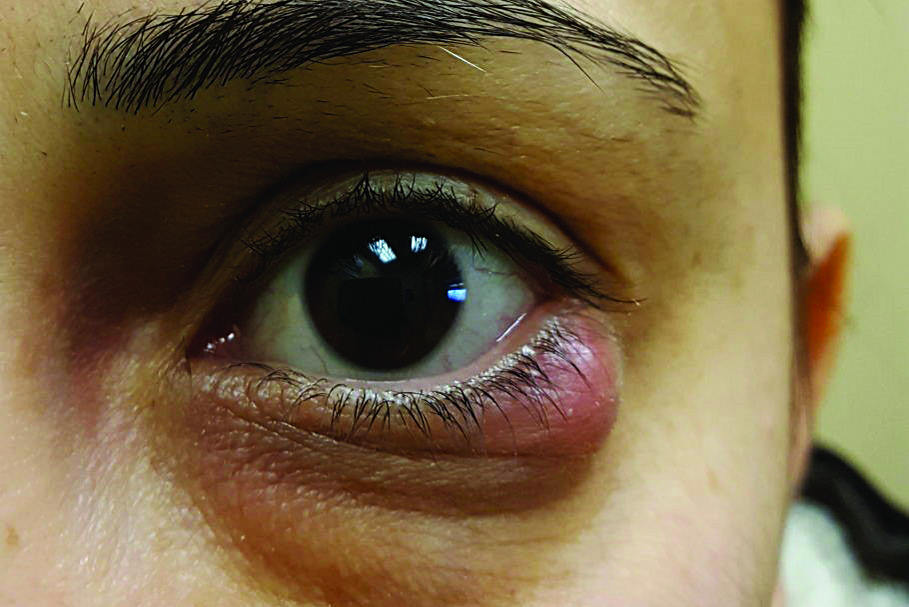
Concern over repeal of Therapeutic Products Act
The Therapeutics Products Act (TPA) 2023 has been repealed, despite being hailed by now-former health minister Dr Ayesha Verrall as the most significant change to the regulation of medicines, medical devices and natural health products in New Zealand in nearly 40 years.
The TPA’s 2013 enactment was also welcomed by the Optometrists and Dispensing Opticians Board (ODOB), who said at the time: “We support the purpose of the TPA, which will help ensure acceptable safety, quality and performance of medicines and medical devices and that there is an evidence base to support any claims of health benefits,” said ODOB’s then-chair Kristine Hammond.
The Act’s repeal, in December last year, is disappointing, said Suzanne Halpin, ODOB chief executive and registrar. “The TPA would have introduced systems and processes to ensure that only safe and high-quality products could be imported and supplied to patients in Aotearoa New Zealand. Overall, the TPA would have bolstered patient safety by ensuring a comprehensive process for the assessment and monitoring of therapeutic products,” she said.
Since the prescribing of spectacles and contact lenses is a restricted activity under the Health Practitioners Competence Assurance Act 2003, the ODOB is concerned about the online supply of spectacles and contact lenses without evidence of a current valid prescription issued by a registered health practitioner, such as an optometrist, said Halpin.
“The regulatory system for medicines, medical devices and natural health products is once again complex and fragmented and is likely to stay that way until new legislation is enacted,” she said. However, the ODOB is hopeful that new legislation will consider current and future practices in the health system to enhance patient safety, she added. “This is the Board’s primary focus. We emphasise to practitioners the importance of being especially vigilant in adhering to safety standards and protocols.”
A recent government statement said the health ministry is developing a new Medical Products Bill to replace the Medicines Act 1981 and that natural health products would be regulated under a standalone bill.