Fun-IVCM reveals inflammation clues in DED
Clinically, dry eye has been broadly classified into two main subtypes: aqueous-deficient and evaporative1. Aqueous-deficient dry eye is characterised by reduced aqueous tear production2 and is often accompanied by ocular surface inflammation3,4. Evaporative dry eye is defined by excessive tear film evaporation, frequently due to tear lipid abnormalities associated with meibomian gland dysfunction5. However, the extent to which inflammation contributes to an individual patient’s dry eye presentation, particularly in cases of mixed aqueous-deficient/evaporative disease, can be challenging to quantify in the clinic.
Bulbar conjunctival hyperaemia can provide a gross, non-specific indication of ocular surface inflammation. Point-of-care testing for elevated levels of matrix metalloproteinase-9 (MMP-9) can identify threshold levels of this pro-inflammatory enzyme in tears, but this immunoassay is not broadly accessible. As such, it can be difficult for a clinician to confidently determine the appropriateness of instituting anti-inflammatory therapy.
In vivo confocal microscopy (IVCM), using the Heidelberg Retinal Tomograph-3 with Rostock Corneal Module, is a clinical technique enabling high-resolution imaging of immune cells in the human cornea7. The presence of inflammation in dry eye disease (DED) has previously been evaluated using this technique, with subsequent analysis of immune cells from static IVCM images. Individuals with aqueous-deficient dry eye have been found to have a higher corneal immune cell density than healthy controls8-10. Dry eye severity has been linked to differences in immune cell shape and morphology, suggestive of alterations to cell activation state and/or function11. However, as static IVCM imaging captures only one snapshot in time, it has limited ability to reliably differentiate subsets of corneal immune cells and cannot be used to evaluate cell behaviours. Assessment of these features, to better define inflammatory responses in the cornea, requires consideration of both a cell’s morphology and dynamic movement.

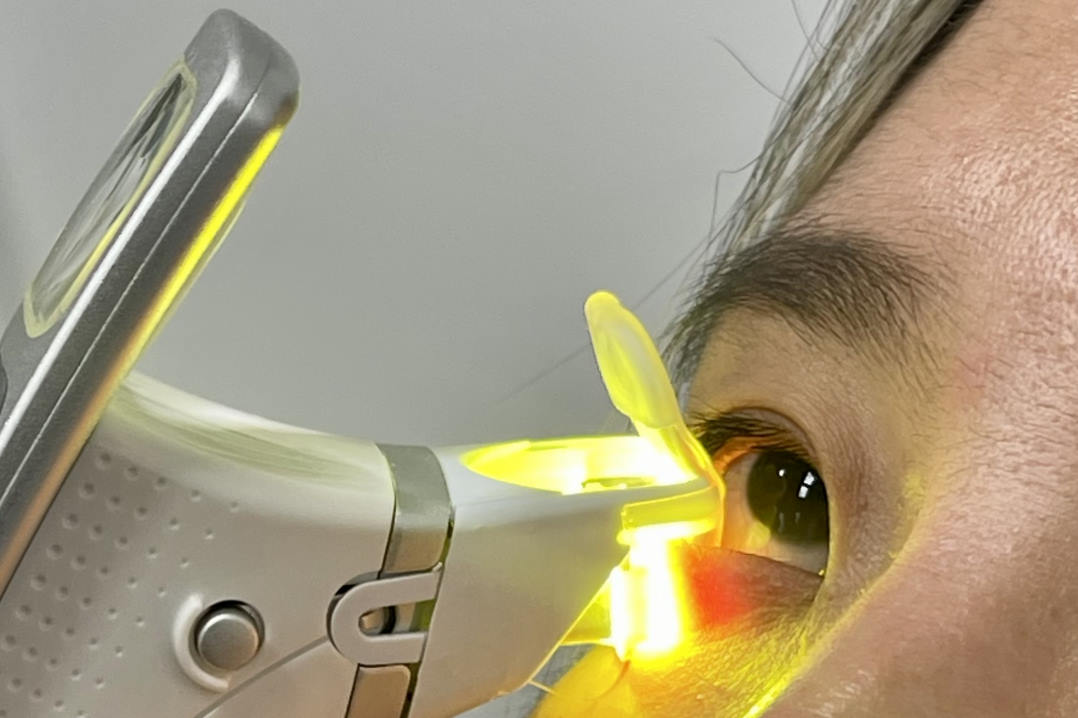
Fig 1 (A). Person undergoing Fun-IVCM corneal imaging. (B) Time-coloured image featuring motile corneal epithelial T cells (arrows); colours identify motile cells, imaged at different timepoints; stationary elements are visible in white. Corneal dendritic cells are relatively less motile and have slender cell bodies, their extendable dendrites survey the tissue area (arrowhead). (C) Time-coloured image showing corneal stromal macrophages (arrowheads), which are relatively stationary during the imaging period but ‘wiggle’ between stationary keratocytes. Scan QR codes to view Fun-IVCM videos
To overcome these limitations, our laboratory developed an imaging approach we term ‘functional in vivo confocal microscopy’ (Fun-IVCM), to track the dynamics of human corneal immune cells in vivo. Through analyses that combined cell morphological and dynamic features, we identified three principal subsets of corneal immune cells in humans: dendritic cells and T cells in the epithelium and macrophages in the stroma12. This finding challenges the dogma that only innate immune cells (dendritic cells and macrophages) exist in the cornea, based on studies in mice, with presumed generalisability to humans. As a result of this advance, we now recognise that both innate and adaptive immune cell subsets can be non-invasively visualised in the human cornea. This new understanding creates avenues to study how these different types of immune cells are affected in ocular conditions, including subtypes of DED, to better define disease pathophysiology and therapeutic care.
Using Fun-IVCM, we recently identified changes to the dynamic activity of corneal dendritic cells and macrophages across different seasons in healthy adults13. These findings suggest there may be differences in the overall inflammatory status of the cornea at different times of the year. In addition, we observed that three hours of soft contact lens wear reduced corneal epithelial T cell numbers and induced greater cell motility12, consistent with a pro-inflammatory tissue response. We have also shown that immunomodulatory medications can return corneal T cell motility to normative levels in people with seasonal allergy12. With this ability to non-invasively quantify subclinical changes to corneal immune cell behaviours in vivo, Fun-IVCM has the potential to be applied to better define corneal inflammation in different forms of DED, as well as to quantify the effects of anti-inflammatory treatments.
Our laboratory has studies underway to investigate how we can leverage Fun-IVCM to define new biomarkers of the in vivo inflammatory status of the cornea and we foresee long-term scope for this research to inform therapeutic patient care.
References
1. Wolffsohn JS, et al. TFOS DEWS III Diagnostic Methodology. American Journal of Ophthalmology, 2025.
2. Stapleton F, et al. TFOS DEWS III Digest Report. American Journal of Ophthalmology, 2025.
3. Pflugfelder SC and CS de Paiva. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology, 2017. 124(11s): p. S4-s13.
4. Sullivan DA.Tearful relationships? Sex, hormones, the lacrimal gland, and aqueous-deficient dry eye. Ocul Surf, 2004. 2(2): p. 92-123.
5. Goto E, et al. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci, 2003. 44(2): p. 533-9.
6. Uchino Y. The Ocular Surface Glycocalyx and its Alteration in Dry Eye Disease: A Review. Invest Ophthalmol Vis Sci, 2018. 59(14): p. Des157-des162.
7. Tuominen IS, et al. Corneal innervation and morphology in primary Sjögren's syndrome. Invest Ophthalmol Vis Sci, 2003. 44(6): p. 2545-9.
8. Kheirkhah A, et al. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Invest Ophthalmol Vis Sci, 2015. 56(12): p. 7179-85.
9. Lin H, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci, 2010. 51(1): p. 122-8.
10. Machetta F, et al. In vivo confocal microscopic evaluation of corneal langerhans cells in dry eye patients. Open Ophthalmol J, 2014. 8: p. 51-9.
11. Aggarwal S, et al. Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. Ocul Surf, 2021. 19: p. 183-189.
12. Downie LE, et al. Redefining the human corneal immune compartment using dynamic intravital imaging. Proceedings of the National Academy of Sciences, 2023. 120(31): p. e2217795120.
13. Wu M, et al. Intravital Imaging of the Human Cornea Reveals the Differential Effects of Season on Innate and Adaptive Immune Cell Morphodynamics. Ophthalmology, 2024. 131(10): p. 1185-1195.


Drs Ching Yi Wu and Mengliang Wu are post-doctoral research fellows in Professor Laura Downie’s research laboratory – the Anterior Eye, Clinical Trials and Research Translation Unit – department of optometry and Vision Sciences, at the University of Melbourne. Ching Yi is an optometrist and clinician scientist whose research focuses on the use of dynamic corneal immune cell imaging to improve understanding of ocular disease. Mengliang is a medical doctor and researcher whose research combines preclinical and clinical methods to advance knowledge of ocular immunology.