Handheld thermal pulsation device for MGD treatment
After successfully navigating Auckland’s extended Covid lockdown in 2021, the Ocular Surface Laboratory (OSL) team was excited to begin its long-awaited clinical trial to evaluate a single thermal pulsation treatment delivered by a novel handheld device (Systane iLux, Alcon) relative to standard-of-care therapy (daily warm compress therapy, followed by lid massage).
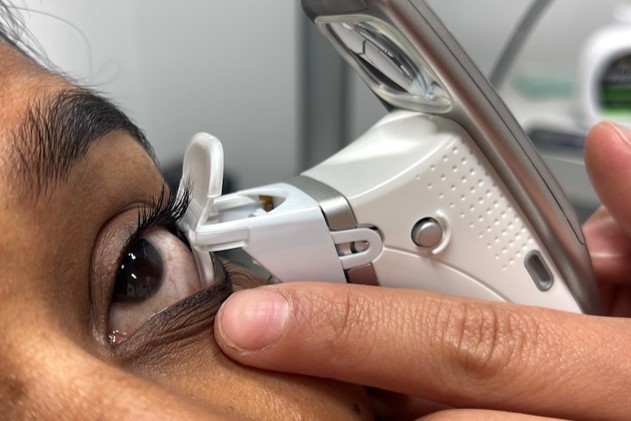
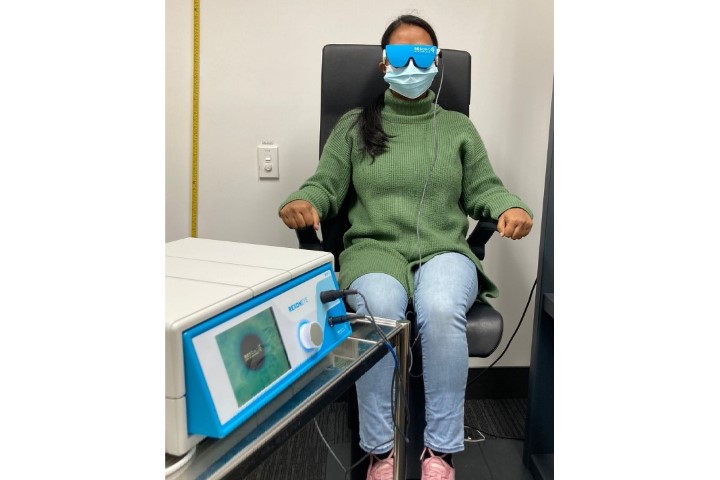
Now commercially available in some parts of the world (though not New Zealand as yet) Systane iLux is a portable, wireless device that allows clinicians to deliver treatment to both eyes of a patient in approximately 8-12 minutes (see Fig 1, top). With this device, clinicians can tailor treatment to the individual by controlling the duration of heat application and the amount of pressure applied when expressing meibum, all while viewing the lids through an incorporated magnifying lens.
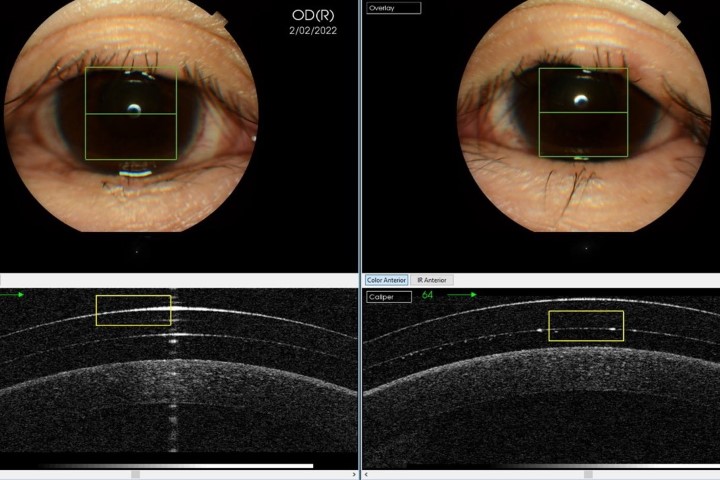
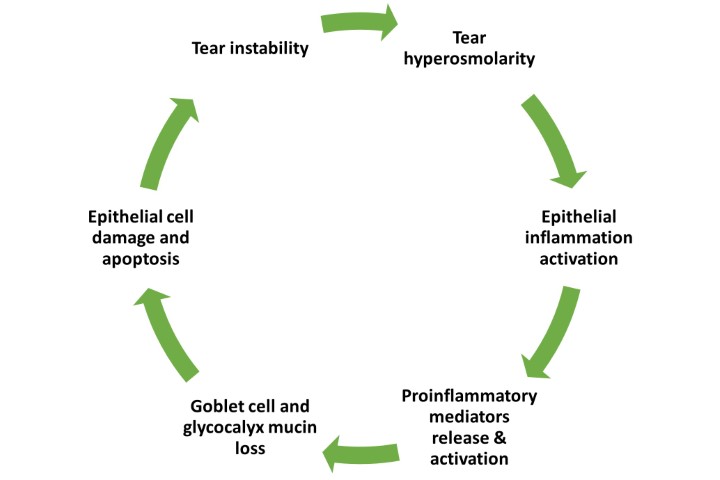
This six-month study was designed as a prospective, investigator-masked, randomised trial. One group (n=38) received a single iLux treatment while the other (n=39) was given a microwavable MGDRx Eyebag (Théa) warm compress1 together with a silicone eyelid massage assistance device (Eyepeace, Cathedral Eyecare)2 for daily application. Participants meeting the TFOS DEWS II diagnostic criteria3 for dry eye disease (DED) and evidence of meibomian gland dysfunction (MGD)4 attended a baseline visit and six-monthly clinical follow-up visits for assessment of symptoms as well as ocular surface and tear film parameters. Efficacy of both treatment modalities was assessed and critical timepoints were identified to help set realistic treatment expectations for both practitioners and patients. Preliminary results established at the three-month time point (described here) were presented at this year’s Association for Research in Vision and Ophthalmology scientific meeting in April in New Orleans.
Three months post-treatment, results confirmed both groups experienced significant and sustained symptomatic improvement. Interestingly, based on the OSDI scores, the iLux group experienced symptomatic relief from month one onwards, whereas it took two months before significant improvement was observed in the control group. Furthermore, a higher percentage of individuals in the iLux group (26%) no longer met the TFOS DEWS II diagnostic criteria3 compared to the control group (17%) at the three-month timepoint. These early study findings suggest thermal pulsation therapy for MGD at a single visit might offer faster symptomatic relief than daily warm compress therapy. Sustained significant improvements in tear film lipid layer grade, expressed meibum quality and lid wiper epitheliopathy were also seen from month one in both groups.
Such symptomatic and clinical improvement in both study groups gives clinicians and patients reassurance that both treatment modalities are effective… if patients are compliant with treatment, and therein, of course, lies the challenge! The good news is that for patients who struggle to adhere to daily self-applied therapy, this single in-office therapy, while admittedly more costly, could present a favourable alternative. Results suggest patients can rapidly regain comfort in their daily routines for an extended period, leaving more time to engage in activities they enjoy. Analysis of the six-month results is underway and expected to provide further insights into the effect duration of this treatment approach. Full outcomes are due to be presented at September 2023's O=Mega/WCO4.
Led by Professor Jennifer Craig, this study received funding from Alcon as an investigator-initiated trial. Alcon had no role in the study design, conduct, analysis, interpretation or reporting of its outcomes.
References
1. Bilkhu P, Naroo S, Wolffsohn J. Randomised masked clinical trial of the MGDRx EyeBag for the treatment of meibomian gland dysfunction-related evaporative dry eye. Br J Ophthalmol. 2014;98(12):1707-11.
2. Wang M, Feng J, Wong J, Turnbull P, Craig J. Randomised trial of the clinical utility of an eyelid massage device for the management of meibomian gland dysfunction. Cont Lens Anterior Eye. 2019;42(6):620-4.
3. Wolffsohn J, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf 2017;15(3):539-74.
4. Tomlinson A, Bron A, Korb D, Amano S, Paugh J, Pearce E, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. IOVS 2011;52(4):2006.

Catherine Jennings is a research optometrist and PhD candidate supervised by Prof Jennifer Craig and Drs Kalika Bandamwar and Alex Müntz in the Ocular Surface Laboratory at the University of Auckland.