NCP drug trial advances
Okyo Pharma has announced plans to begin a phase 2 trial of OK-101, a non-opioid analgesic developed to reduce neuropathic corneal pain (NCP).
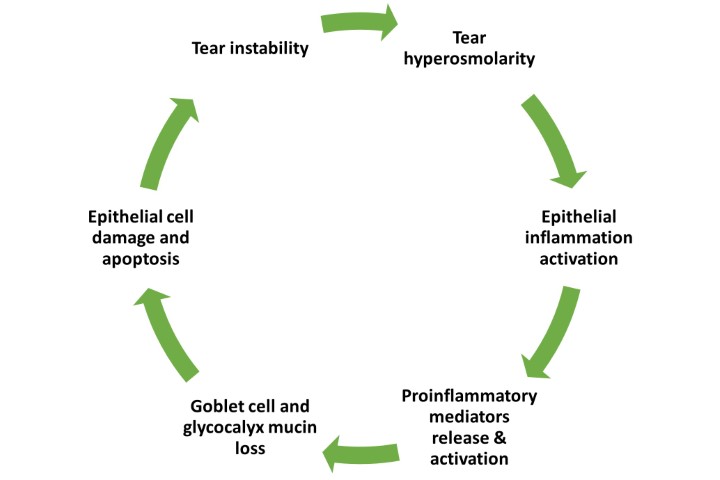
“NCP, which can exhibit as a severe, chronic or debilitating condition in patients suffering from a host of ophthalmic conditions, is presently treated by various topical and systemic treatments in an off-label fashion,” said study lead Professor Pedram Hamrah, director of the Center for Translational Ocular Immunology at Tufts Medical Center, US. “However, there are no approved commercial treatments currently available for this condition.”
Okyo said it will file an Investigational New Drug (NDA) application for OK-101 later this year, with study enrolment expected to commence once this is approved by the FDA.